April 20, 2020
There at least 78 coronavirus vaccines, five of which are in clinical trials. This is where we stand right now in the development of COVID-19 vaccines.
As of today, COVID-19 vaccine research and development includes 115 candidates worldwide (Fig. 1), with 78 confirmed projects and 37 unconfirmed (information on their development cannot be found in available online sources). Of the 78 projects, 73 are currently at early stages of development. The most advanced candidates have recently moved into clinical development, including mRNA-1273 from Moderna, Ad5-nCoV from CanSino Biologicals, INO-4800 from Inovio, LV-SMENP-DC and pathogen-specific aAPC from Shenzhen Geno-Immune Medical Institute (Table 1). Other vaccines will soon follow suit. The unprecedented global rapidity of the research and development is being closely monitored by key authorities, such as the World Health Organization (WHO), to keep us informed and updated on its evolution, failures, and successes.
From the analyst's couch on 9 April 2020. The COVID-19 vaccine development landscape.
DOIi: 10.1038/d41573-020-00073-5
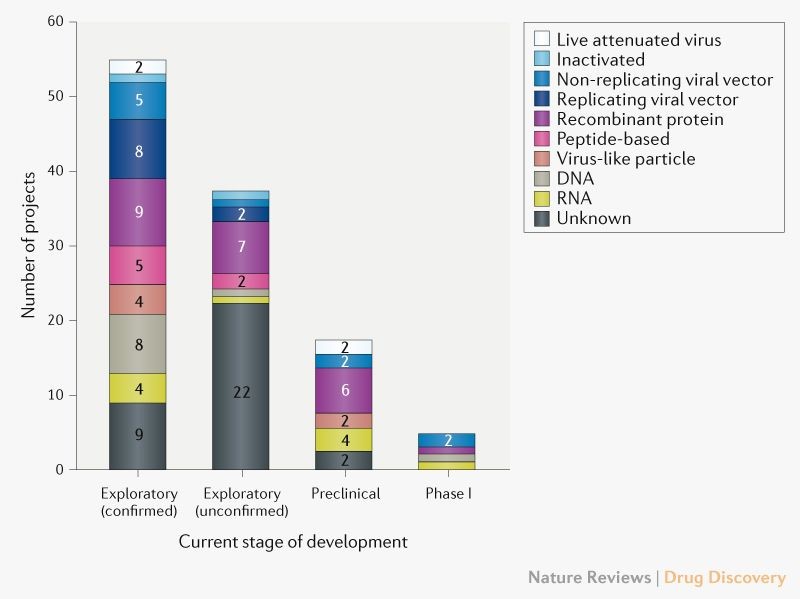
Fig. 1 | Pipeline of COVID-19 vaccine candidates by technology platform. Exploratory projects (split into confirmed and unconfirmed) are in the early planning stage with no in-vivo testing, and preclinical projects are at the stage of in-vivo testing and/or manufacturing clinical trials material.
Luciana Aparecida Campos, Ph.D.
College of Health Sciences
Abu Dhabi University

